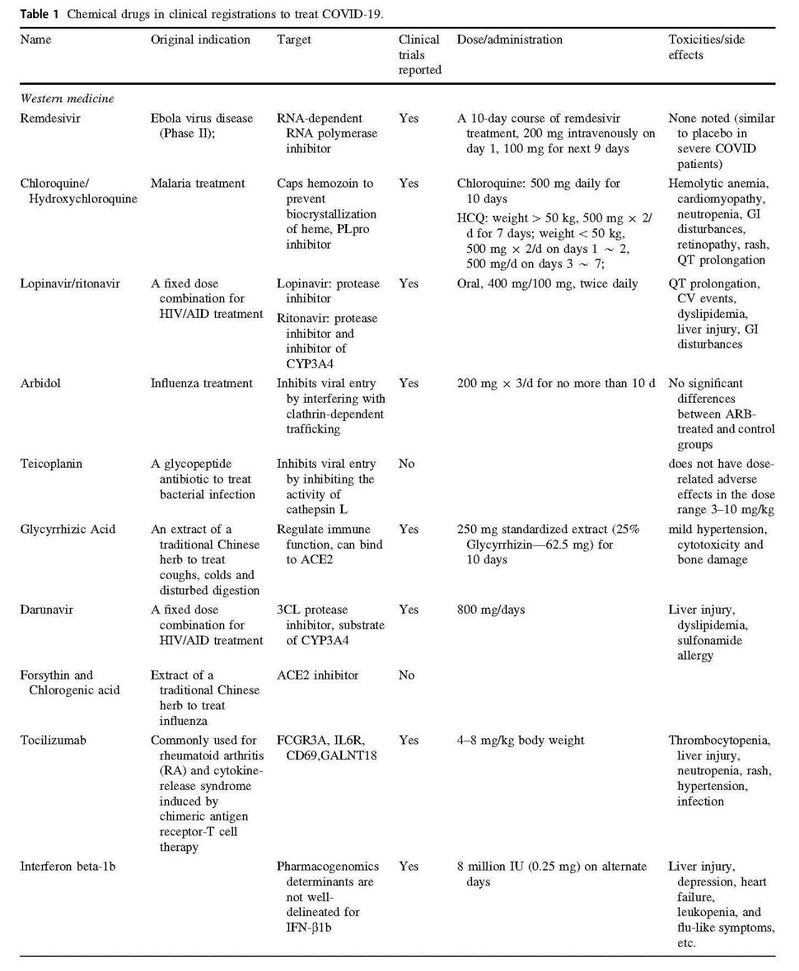
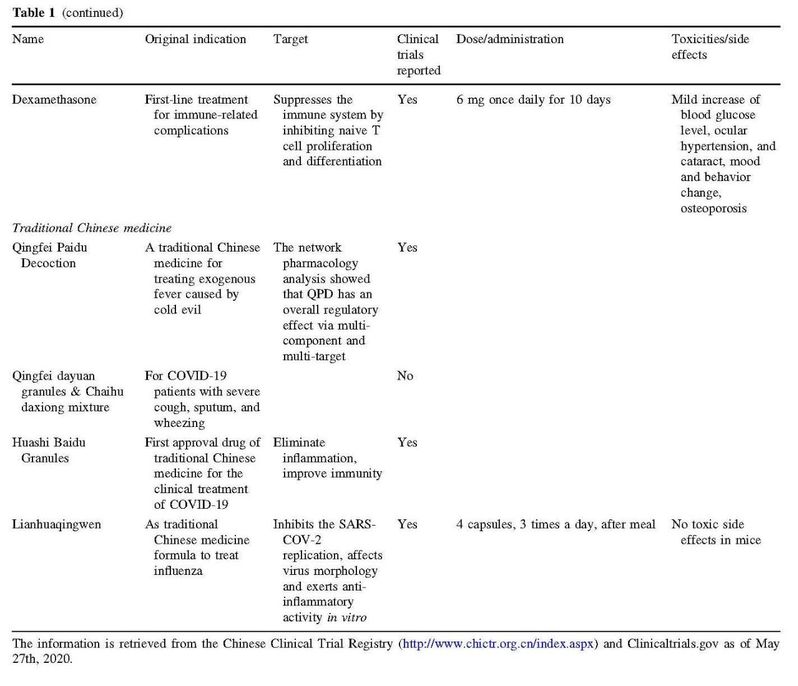
近日,国际学术期刊《Virologica--Sinica》在线发表了我院张其威教授研究组题为"COVID-19: Antiviral Agents, Antibody Development and Traditional Chinese Medicine"的文章,本文介绍了全世界有发展前景的新冠病毒治疗药物,包括现存的抗病毒药物的老药新用、基于网络的药理学研究、抗体药物和传统中药研发。部分药物已进入最后一个临床试验阶段或已批准用于临床新冠病毒治疗。该综述文章对比分析新冠病毒各种治疗药物实验室和临床治疗效果,有助于促进新冠治疗药物的研发和改进,并为临床治疗和应用提供重要参考。

摘要:世界卫生组织(WHO)宣布2019年底出现的由新型冠状病毒SARS-CoV-2引起的冠状病毒疾病(COVID-19)是第一个冠状病毒全球大流行。目前世界上还未出现有效的抗SARS-CoV-2药物获批用于治疗COVID-19患者,因此迫切需要提供多种治疗选择应对新冠病毒疾病暴发。为促进新冠肺病毒治疗药物的更好和更快发展,并为临床治疗提供参考意见,我们在此介绍一些有发展前景的治疗药物,包括现存的抗病毒药物的老药新用、基于网络的药理学研究、抗体开发和中药发展。其中部分药物已进入最后一个临床试验阶段。
Abstract: The World Health Organization (WHO) has declared coronavirus disease 2019 (COVID-19) is the first pandemic caused by coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, there is no effective anti-SARS-CoV-2 drug approved worldwide for treatment of patients with COVID-19. Therapeutic options in response to the COVID-19 outbreak are urgently needed. To facilitate the better and faster development of therapeutic COVID-19 drugs, we present an overview of the global promising therapeutic drugs, including repurposing existing antiviral agents, network-based pharmacology research, antibody development and traditional Chinese medicine. Among all these drugs, we focus on the most promising drugs (such as favipiravir, tocilizumab, SARS-CoV-2 convalescent plasma, hydroxychloroquine, Lianhua Qingwen, interferon beta-1a, remdesivir, etc.) that have or will enter the final stage of human testing—phase III–IV clinical trials.
参考文献:
Wenyi Guan, Wendong Lan, Jing Zhang, Shan Zhao, Junxian Ou, Xiaowei Wu, Yuqian Yan, Jianguo Wu and Qiwei Zhang. COVID-19: Antiviral Agents, Antibody Development and Traditional Chinese Medicine. Virologica Sinica. 2020: 10.1007/s12250-12020-00297-12250.